All formulas of chapter some basic concepts of chemistry Brainly.in

That's great! But even if you are not, you must know some basic concepts of Chemistry. I'm sure after learning these, you might become a fan of Chemistry. Let's learn about what Chemistry actually is and it's basic concepts that will help you understand Chemistry a lot better. Atomic Mass and Molecular Mass. Concentrations. Dalton's.
Chemistry Formulas Cheat Sheet Teaching chemistry, Chemistry notes

Unit 1 Some BaSic conceptS of chemiStry Science can be viewed as a continuing human effort to systematise knowledge for describing and understanding nature. You have learnt in your previous classes that we come across diverse substances present in nature and changes in them in daily life.
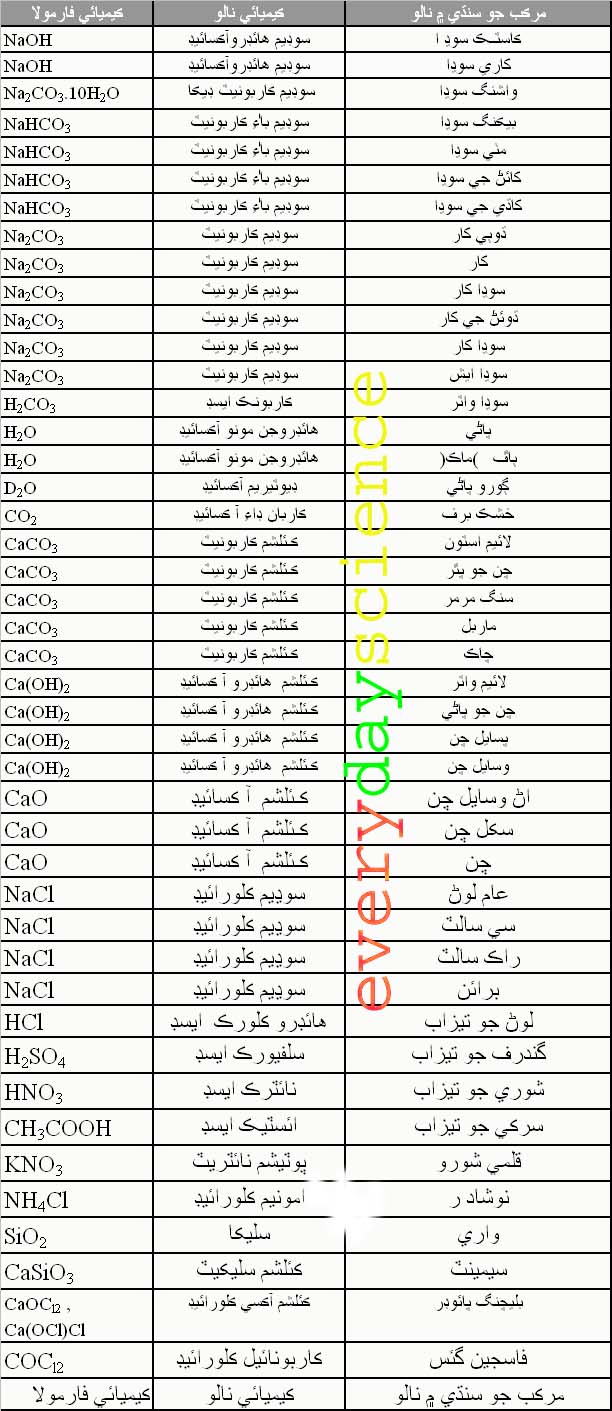
Everyday Science CHEMICAL FORMULAS
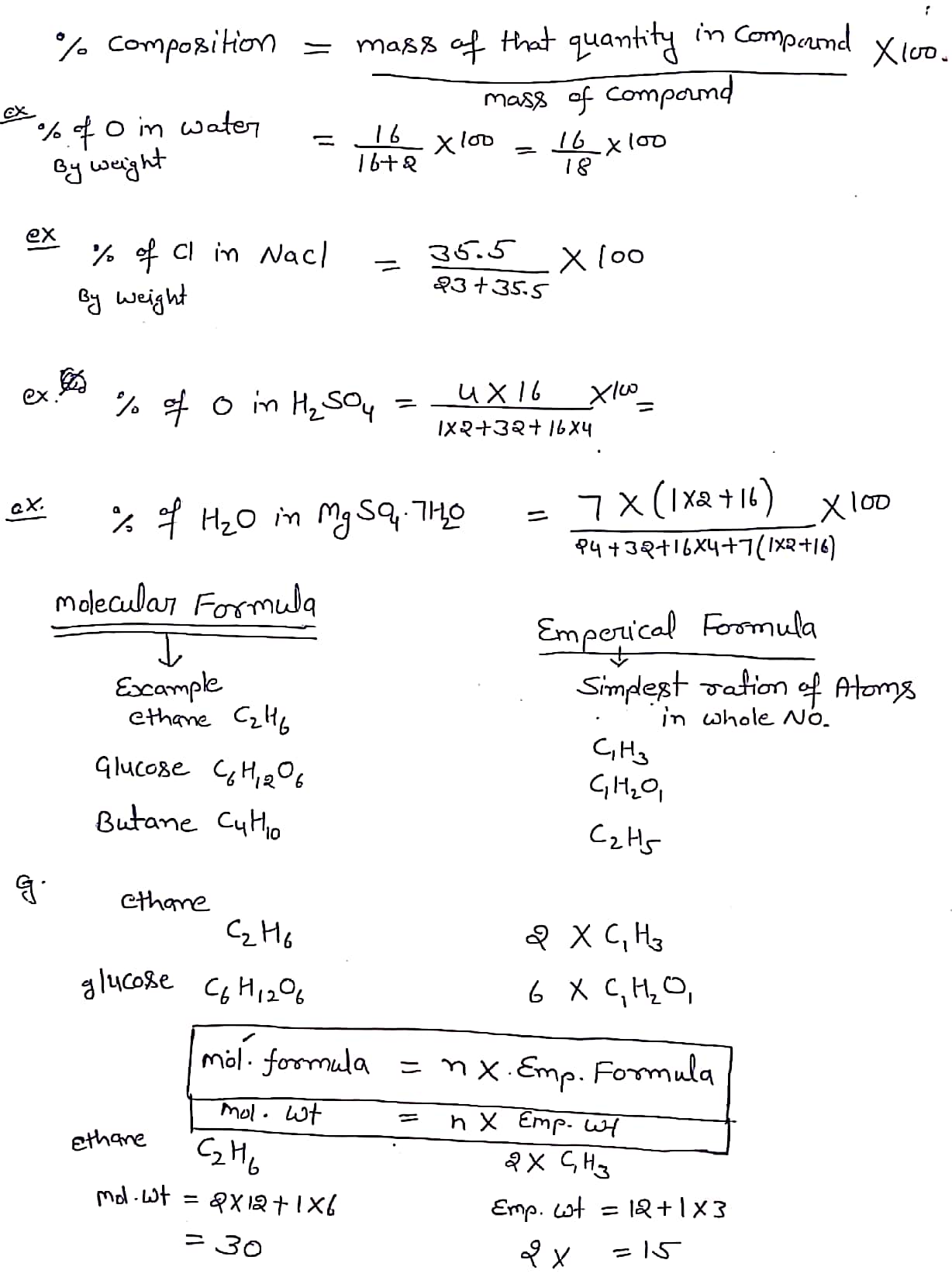
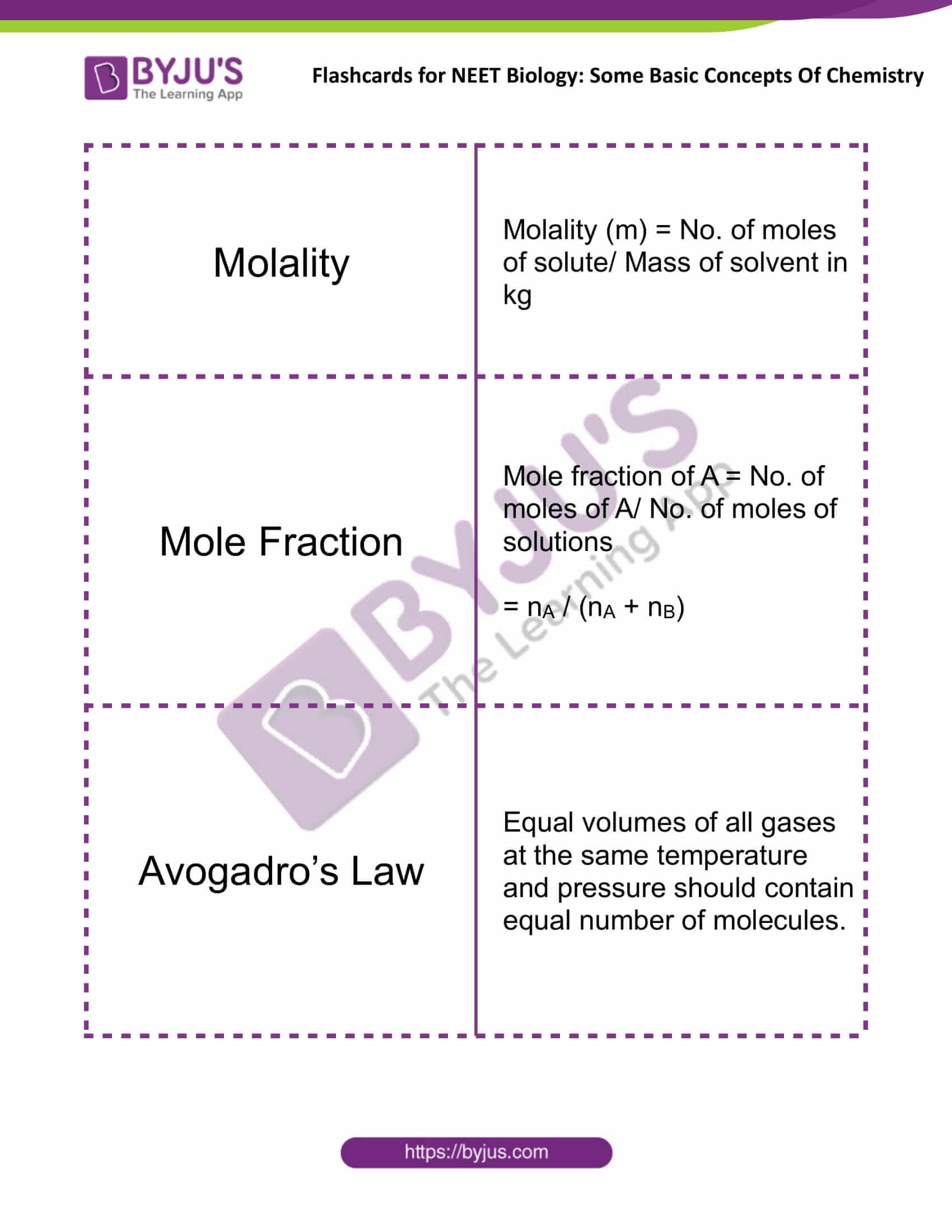
Solution Number of molecules in W (g) of substance = W × N A M ( Molecular mass) Molality m = No. of moles of solute Mass of solvent ( kg) Number of molecules in V litres = V × N A 22. 4 L of gas at STP Number of gram atoms = W G AM ( gram atomic mass) Number of gram molecules = W G MM ( gram molecular mass)
Some basic concepts of chemistry notes Studypur
Molecules represent the basic unit of a chemical compound, and this another of those essential basic chemistry concepts. That's basically it. An example is a water molecule, which is made from two atoms of hydrogen (H) and one atom of oxygen (O), held together via covalent bonds. Models of a water molecule.
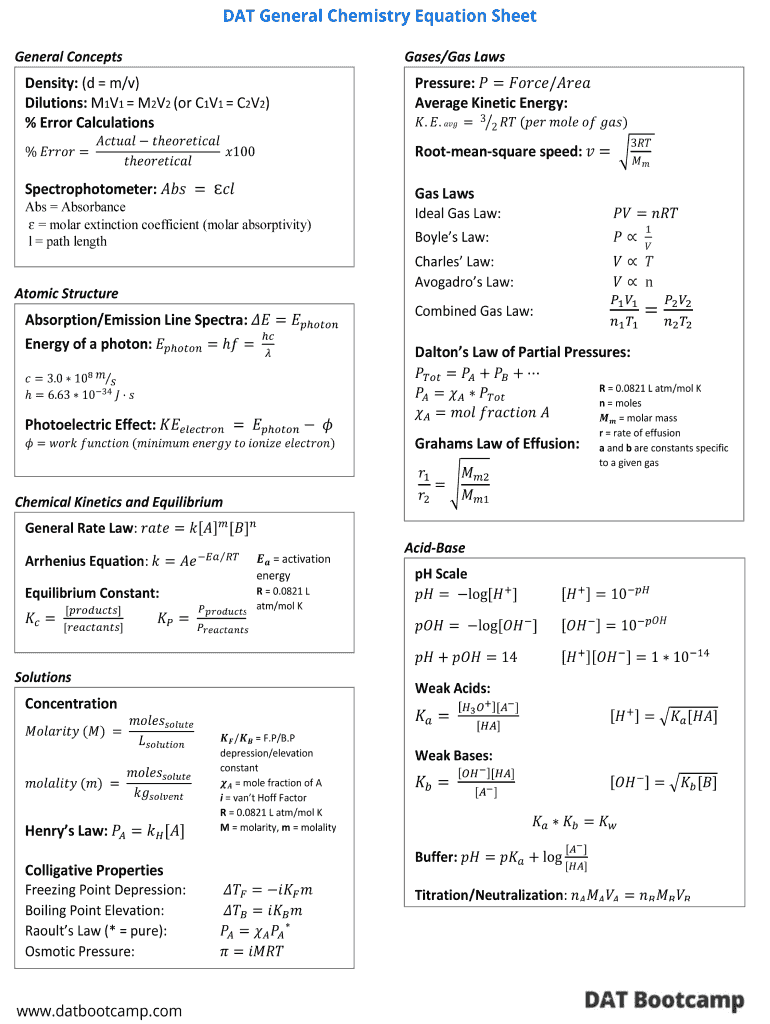
Ap Chem Formula Sheet Fill Out and Sign Printable PDF Template signNow
The attached handbook has all chapters which will come in the NEET exam for NEET Chemistry Formula Handbook for all Chapters. It has full details of all formulas which are applicable in all chapters. The handbook has been designed by NEET teachers and all formulas have been given chapter wise. Please see the list of chapters below for which you.
Some basic concept of chemistry Class 11 formula PW

The metal, having lost one or more electrons, forms a cation, an ion with a positive charge; the nonmetal, having gained one or more electrons, becomes an anion, an ion with a negative charge. When two elements form a covalent bond, one or more electron pairs are shared between these two elements.
Molecules, Ions, and Chemical Formulas
This video includes detailed explanation of atomic, molecular and formula mass of chapter 1 (Some Basic Concepts of Chemistry)If you like our work, then you.
Some basic concept of chemistry Class 11 formula PW

Chemistry formula for class 11 chapter- some basic concept of chemistry Jul 05, 2022, 16:45 IST Matter is substance which has mass and occupies space, e.g., water, air, box, table, etc. State of Matter: Solid, liquid and gaseous state represent three different states of mater.
Pin on dvpmt perso

'Some basic concepts of chemistry' is the most fundamental chapter of complete chemistry. It gives information about the atomic number and mass number of elements.
Mind map of Chapter 1 SOME BASIC CONCEPT OF CHEMISTRY class 11th

Solution. The H:C ratios for the two alcohols are 4:1 = 4.0 for methanol and 6:2 (3.0) for ethanol. Alternatively, one sometimes uses mole fractions to express the same thing. The mole fraction of an element M in a compound is just the number of atoms of M divided by the total number of atoms in the formula unit.
CBSE Class 11 Some Basic Concepts of Chemistry FORMULAE Concepts for
"Some Basic Concepts of Chemistry" is the first chapter in the Class 11 Chemistry syllabus as prescribed by NCERT. The chapter touches upon topics such as the importance of Chemistry, atomic mass, and molecular mass.
Pin by Joseph Quattrocchi on Phillips Academy Chemistry review

In this lecture, I will teach you the full chapter of some basic concepts of chemistry class 11. You will learn all the important topics of basic concepts of.
Chemistry Formulas Chart HSC Higher Secondary Education Website

Some basic Concepts of Chemistry | Khan Academy Physical Chemistry (Essentials) - Class 11 8 units · 52 skills Unit 1 Welcome to physical chemistry Unit 2 Structure of atom Unit 3 Some basic Concepts of Chemistry Unit 4 Redox reactions Unit 5 Gaseous state Unit 6 Thermodynamics Unit 7 Chemical Equilibrium Unit 8 Ionic equilibrium Course challenge
Introduction to some basic concepts of chemistry YouTube

Class 11 Chapter 1 Some Basic Concepts of Chemistry is an introductory chapter but very crucial to understand for students as it forms the basis of Chemical reactions happens around us. To understand these concepts, Class 11 Chapter 1 Chemistry Notes are prepared by subject experts in well-defined and easy language.
Some Basic Concepts of Chemistry Flashcards for NEET Chemistry
Answer: (i) Molecular mass of H 2 O = 2 (1.008 amu) + 16.00 amu=18.016 amu (ii) Molecular mass of CO 2= 12.01 amu + 2 x 16.00 amu = 44.01 amu (iii) Molecular mass of CH4= 12.01 amu + 4 (1.008 amu) = 16.042 amu Question 2. Calculate the mass percent of different elements present in sodium sulphate (Na2 SO4). Answer: More CBSE Class 11 Study Material
Chemistry formula for class 11 chapter some basic concept of chemistry

Some basic concepts of chemistry | Khan Academy Class 11 Chemistry (India) 13 units · 107 skills Unit 1 Some basic concepts of chemistry Unit 2 Structure of atom Unit 3 Classification of elements & periodicity in properties Unit 4 Chemical bonding and molecular structure Unit 5 States of matter Unit 6 Thermodynamics Unit 7 Equilibrium