Pin on Chemistry

This sequence of color changes will repeat with a period of approximately 15 seconds at 25 oC. The reaction last about 5 min. The Briggs-Rauscher Reaction. IO3- + 2 H2O2 + CH2(CO2H)2 + H+ --> ICH(CO2H)2 + 2 O2 + 3 H2O (1) This reaction can be broken into two component reactions:
BriggsRauscher Tepkimesi (The BriggsRauscher Reaction) YouTube

The oscillations of the Briggs-Rauscher reaction depend on the presence of free radicals in the solution. Adding antioxidants has been reported to change the oscillations due to their ability to "scavenge" the free radicals. Devise a way to use the Briggs-Rauscher reaction to test a chemical's ability to function as an antioxidant.
Black & White Magic (BriggsRauscher Reaction) YouTube

The Briggs-Rauscher oscillating reaction is one of a small number of known oscillating chemical reactions. It is especially well suited for demonstration purposes because of its visually striking color changes: the freshly prepared colorless solution slowly turns an amber color, suddenly changing to a very dark blue.
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
The Briggs-Rauscher reaction is an oscillating clock chemical reaction that cycles from clear, amber, to deep blue several times before finally ending as a blue-black mixture. It is one of the most popular chemistry demonstrations because it's easy and reliable. How to Do the Briggs-Rauscher Reaction Doing the Briggs-Rauscher reaction is simple.
X870 BriggsRauscher Reaction Oscillating Clock Lecture Demonstration Manual General

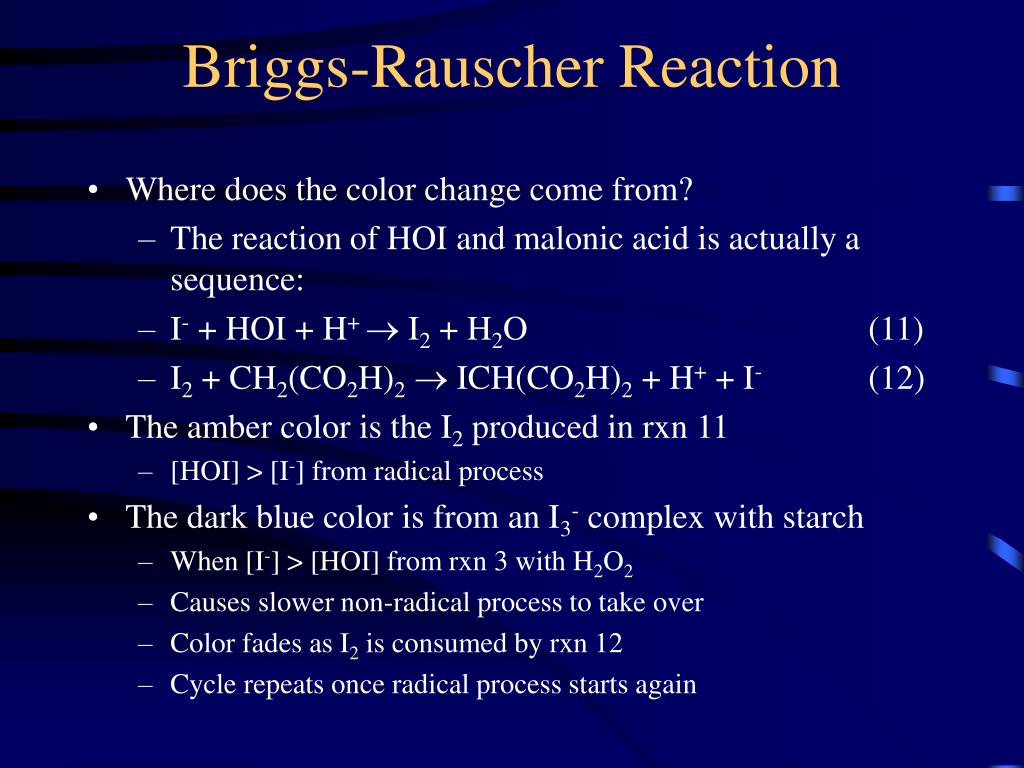
The reason for this is that in the first reaction, molecular iodine forms, which gives the solution an amber color. The iodine then turns into triiodide ions, which bond with starch and form a complex compound of a dark blue color. Parallel with this process, a reaction takes place between molecular.
BriggsRauscher Reaktion Flaskman

ABSTRACT: The Briggs Rauscher reaction is a popular − demonstration to illustrate chemical oscillations in laboratories, classrooms, and public seminars because of its simplicity and visual appeal. Here, we adapt the Briggs Rauscher reaction to present − reaction diffusion convection patterns in the undergraduate − general or physical.
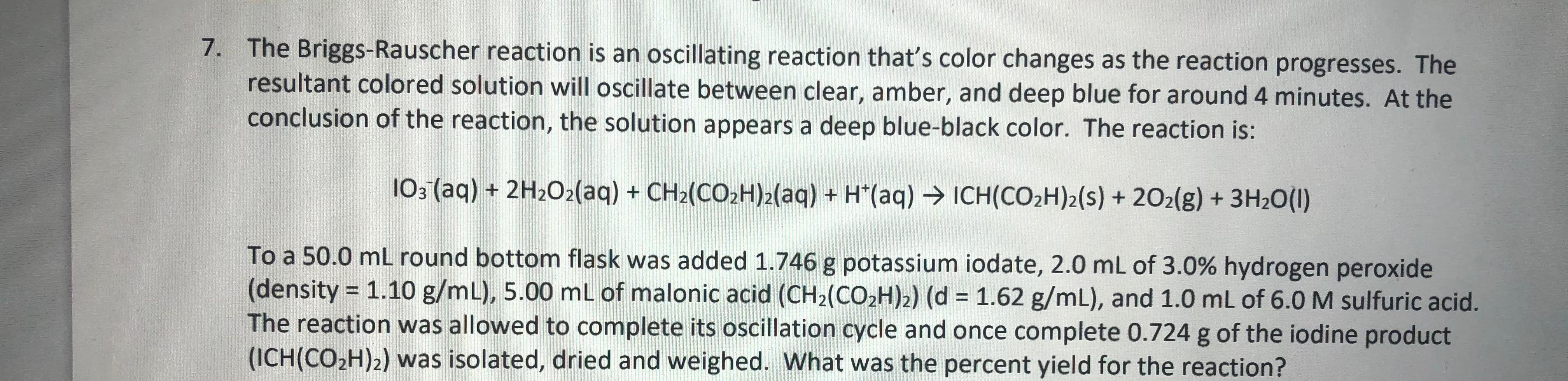
Solved 7. The BriggsRauscher reaction is an oscillating
There's something about an oscillating reaction that really makes students' jaws drop and gets them asking questions. Among the many types of such reactions,.
Briggs Rauscher Reaction ChemTalk

The Briggs-Rauscher reaction, also known as 'the oscillating clock', is one of the most common demonstrations of a chemical oscillator reaction. The reaction begins when three colorless solutions are mixed together. The color of the resulting mixture will oscillate between clear, amber, and deep blue for about 3-5 minutes.
BriggsRauscher Reaction YouTube

How To Do Briggs-Rauscher Reaction At Home DiyDon't Forget To Subscribe UsWebsite: http://diyfactoryworld.blogspot.com/Like Facebook: https://.
Briggs Rauscher Oscillating Chemical Reaction YouTube

This is the Briggs Rauscher reaction, the coolest chemistry demonstration on the planet. This is an oscillating reaction, which means the reactants will form products, which will reform the reactants several times, until one of the reactants is extinguished and the reaction stops. The blue color is an iodine-starch compound.
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379

The Briggs-Rauscher reaction, also known as 'the oscillating clock', is one of the most common demonstrations of a chemical oscillator reaction. The reaction.
Simulated behaviour of [I À ] vs. time of the acetonebased BR... Download Scientific Diagram

Womble Bond Dickinson Places 20 Attorneys on 2024 North Carolina Legal Elite. jan 03 2024.
PPT Chemistry of Oscillating ColorChanging Reactions PowerPoint Presentation ID582379
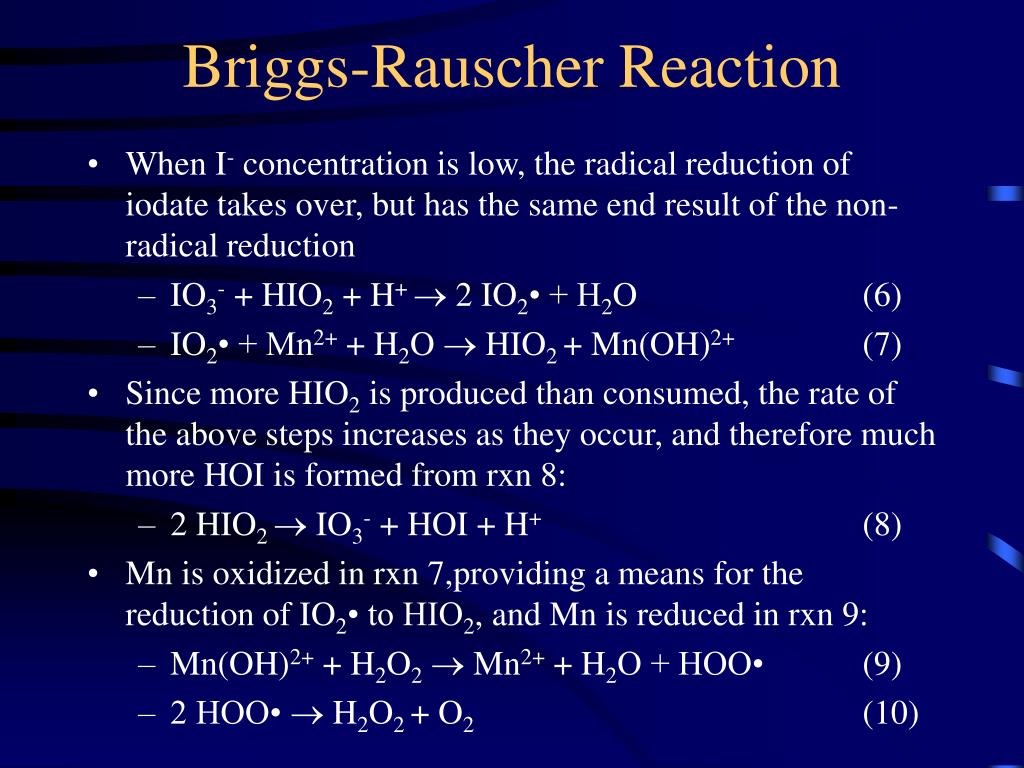
B ("radical process"): A fast process involving manganese and free radical intermediates, which converts hydrogen peroxide and iodate to free iodine and oxygen. This process also can consume iodide up to a limiting rate. IO 3- + 2 H 2 O 2 + CH 2 (CO 2 H) 2 + H + -> ICH (CO 2 H) 2 + 2 O 2 + 3 H 2 O. The striking visual demonstration occurs by.
BriggsRauscher Reaksiyonu Kimyaca

10) the defendant shall permit a probation officer to visit him or her at any time at home or elsewhere and shall permit confisc ation of any contraband observed in plain view of the probation officer; 11) the defendant shall notify the probation officer within sevent y-two hours of being arrested or questioned by a law enforceme nt officer;
BriggsRauscher reaction Alchetron, the free social encyclopedia

While there are a variety of such reactions out there, perhaps the most iconic is the Briggs-Rauscher reaction. The rotating orange-black-colourless changes are incredibly reliable and the solutions, once made up, can tolerate a wide spectrum of concentrations.
How To Do BriggsRauscher Reaction At Home Diy YouTube

The Briggs-Rauscher oscillating reaction is one of the very few oscillating reactions that we know of. Throughout the reaction, the concentration of iodide (.